Oral treatment for spinal muscular atrophy, a genetic neurological disorder, now available in India
Spinal muscular atrophy, a life-threatening genetic disorder, affects one in 7,744 live births in India and is the leading genetic cause of infant mortality in the country. Evrysdi, the first oral treatment for this disorder, launched today in India.


Depending on the type of this neurological disorder, an individual’s physical strength and their ability to walk, eat or breathe can be significantly diminished or lost. Photo: freepik.com
Patients suffering from spinal muscular atrophy, a life-threatening neuromuscular disorder, can now avail an oral treatment in India. This is the first time such a treatment for the disorder has been launched in the country.
Evrysdi was launched today on July 29, by the world’s largest biotech company, Roche Products Private Limited. Based in Basel, Switzerland, the biotech company offers medicines in infectious diseases and diseases of the central nervous system.
“Today heralds a new journey of hope as we are all coming together to add color into the lives of SMA [spinal muscular atrophy] patients in India with the launch of Evrysdi,” said V Simpson Emmanuel, chief executive officer and managing director of Roche Pharma India, during the virtual launch.
“We believe no patient should be deprived an opportunity to live a healthy life, however complex or rare the disease is,” he added. Roche is committed to making a difference to the lives of people in India, he said.
Also Read: The lockdown has increased vulnerabilities of people with disabilities
Leading cause of child mortality
Experts in the virtual launch informed that the disease affects one in 7,744 live births in India and is the leading genetic cause of infant mortality in the country. Most infants are unable to survive beyond a few years.
A majority of people with this life-threatening neuromuscular disease in India remain untreated. “Given the majority of people with SMA in India remain untreated, we believe Evrysdi, with its highly efficacious clinical profile and oral administration advantage, will offer meaningful benefits for many living with this rare neurological disease,” said Bruno Jolain, Chief Medical Officer of Roche Pharma India.
“Administration of Evrysdi requires no hospitalization, no anesthesia, no specialized care center, no complex administration and no steroids. A simple oral administration gives SMA patients, treating physicians and caregivers more control over their daily lives,” he added.
How does spinal muscular atrophy affect a person?
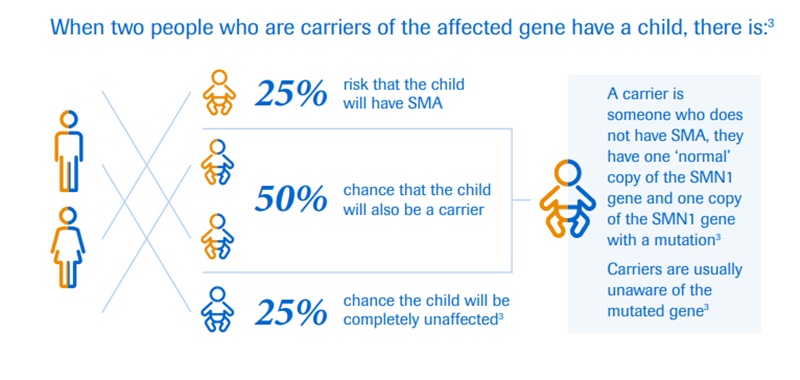
Spinal muscular atrophy is a whole-body genetic disorder that causes muscle weakness throughout the body. It is caused by a mutation of the survival motor neuron gene, which leads to a deficiency of survival motor neuron (SMN) protein. This protein is found throughout the body and is essential to the function of nerves that control muscles and movement. Without it, nerve cells cannot function correctly causing muscle weakness over time.
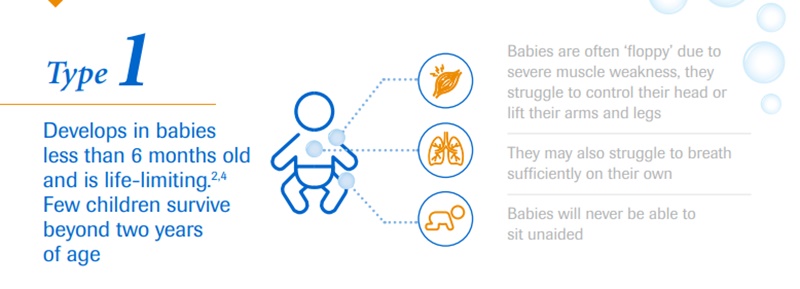
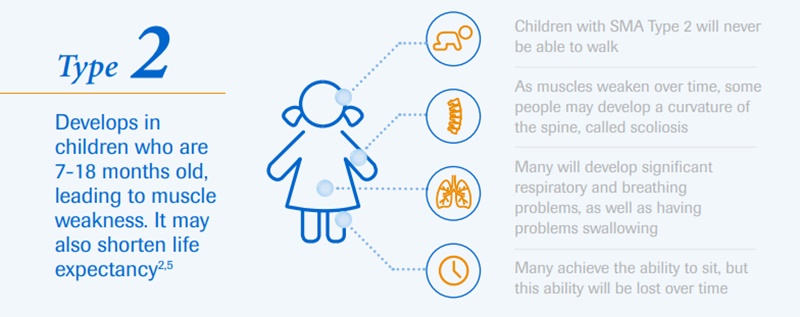
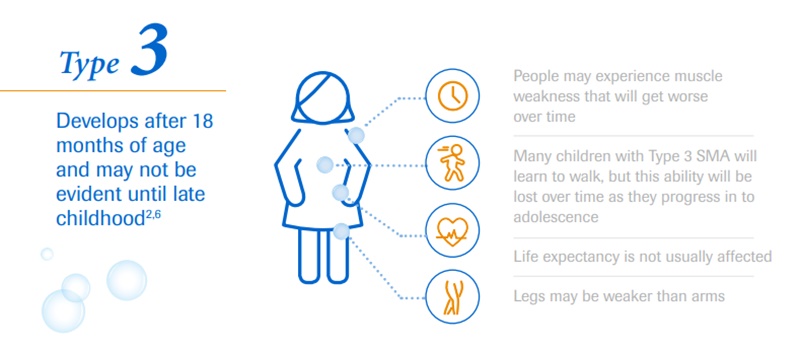
The affected kids develop muscular weakness, which initially affects their upper and lower limbs. However, over time they develop difficulty in breathing and swallowing.
Depending on the type of this neurological disorder, an individual’s physical strength and their ability to walk, eat or breathe can be significantly diminished or lost. It is a severe, progressive rare neuromuscular disease that can be fatal.
Oral treatment: Rs 600,000 per bottle
As per the company’s press statement, Evrysdi can be administered daily at home orally. “Evrysdi is supplied as powder which is constituted into a liquid solution and taken once daily by mouth or feeding tube if required. It is designed to treat SMA by increasing production of the Survival Motor Neuron (SMN) protein,” read the statement.
The biotech company has also announced a patient support programme for Evrysdi so that every patient gets access to this disease modifying treatment. Priced at Rs 600,000 a bottle, Roche has claimed to provide three bottles free for every two bottles bought by the patient in the first two years of treatment through the programme.
From the third year onwards, Roche has claimed it will provide two bottles free for every one bottle bought by the patient.
During the virtual launch, Emmanuel informed that an infant weighing up to five kilogrammes (kgs) will require one bottle for 60 days whereas those up to 20 kgs will require one bottle for 12 days.
The oral treatment was first approved by the United States Food and Drug Administration in August last year, and is today available in India. Evrysdi was approved by Indian health authorities after reviewing its efficacy and safety data from global clinical studies, informed the biotech company.
The company also claimed that over 4,000 patients in at least 50 countries worldwide have benefitted from Evrysdi.

Home delivery for the treatment
As spinal muscular atrophy patients have weak pulmonary health, they find it difficult to visit a hospital to receive the treatment. Besides, motor disability adds to the burden for both the patients and their caregivers.
In order to address this issue, Roche has claimed to provide free home delivery of Evrysdi to each and every patient following consent from the patient, caregiver and their healthcare practitioners.
Roche can be reached out at a toll free number (1800-202-4755) for additional information regarding Evrysdi’s availability and free home delivery.

