COVID19 vaccines ‘may need to be updated’: WHO body
A technical advisory group established by WHO suggests the current COVID19 vaccines need to elicit immune responses that are “broad, strong, and long-lasting” in order to reduce the need for successive booster doses.

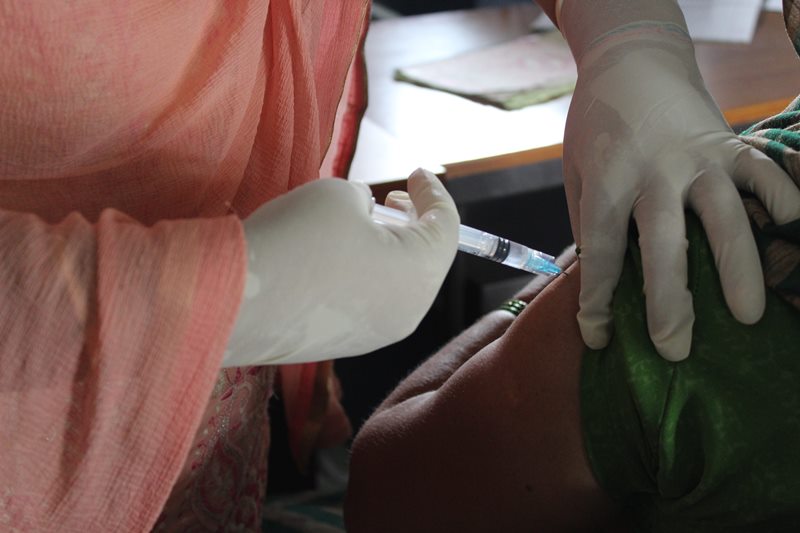
The technical advisory group has recommended that COVID19 vaccines need to be more effective against infection. Photo: Gaon Connection
A Technical Advisory Group on COVID19 Vaccine Composition (TAG-CO-VAC) established by the World Health Organization (WHO) has suggested that the current COVID19 vaccines “may need to be updated” to ensure they are effective against new variants such as Omicron.
“COVID-19 vaccines that have high impact on prevention of infection and transmission, in addition to the prevention of severe disease and death, are needed and should be developed,” the WHO body stated on January 11.
“Until such vaccines are available, and as the SARS-CoV-2 virus evolves, the composition of current COVID-19 vaccines may need to be updated, to ensure that COVID-19 vaccines continue to provide WHO-recommended levels of protection against infection and disease by VOCs [Variant of Concerns], including Omicron and future variants,” it stated.
The WHO’s technical advisory group reviews and assesses the public health implications of emerging SARS-CoV-2 Variants of Concern (VOC) on the performance of COVID19 vaccines. It also recommends WHO on COVID19 vaccine composition.
The technical advisory group has recommended that COVID19 vaccines need to be more effective against infection which will lower community transmission. It also recommended that COVID19 vaccines need to elicit immune responses that are “broad, strong, and long-lasting” in order to reduce the need for successive booster doses.
Also Read: Explained: For how long do the vaccines offer protection against COVID19?
Two days ago, on January 10, India began administering the third dose of COVID19 vaccine as the ‘precaution’ dose to healthcare workers, front line workers, and people aged 60 years and above with comorbidities who have received two doses of COVID19 vaccine.
Also Read: No need for new registration for ‘precaution’ dose of COVID19 vaccine: Centre
The ‘precaution’ vaccine dose will be the same as the first two doses received by the beneficiaries. For instance, those who received Covaxin will receive Covaxin while those who were inoculated with Covishield will receive a Covishield booster dose.
In the past two days, over two million (2,325,264) ‘precaution’ doses have been administered to eligible beneficiaries in India. The country is currently administering three COVID19 vaccines — Serum Institute of India’s Covishield, Bharat Biotech’s Covaxin and Russian-made Sputnik V — for its vaccination drive.
The technical advisory group has suggested that “a vaccination strategy based on repeated booster doses of the original vaccine composition is unlikely to be appropriate or sustainable.”
Meanwhile, the technical advisory group has suggested following options to consider:
- A monovalent vaccine that elicits an immune response against the predominant circulating variant(s), although this option faces the challenge of the rapid emergence of SARS-CoV-2 variants and the time needed to develop a modified or new vaccine.
- A multivalent vaccine containing antigens from different SARS-CoV-2 variants of concerns.
- A pan SARS-CoV-2 vaccine: a more sustainable long-term option that would effectively be variant-proof.

