Bombay High Court directs the Central govt to compulsorily procure two patented TB drugs
India has the world’s highest burden of tuberculosis. TB patients face shortage of two patented drugs -- Bedaquiline and Delamanid. The recent high court order may provide the much needed relief to the TB patients.

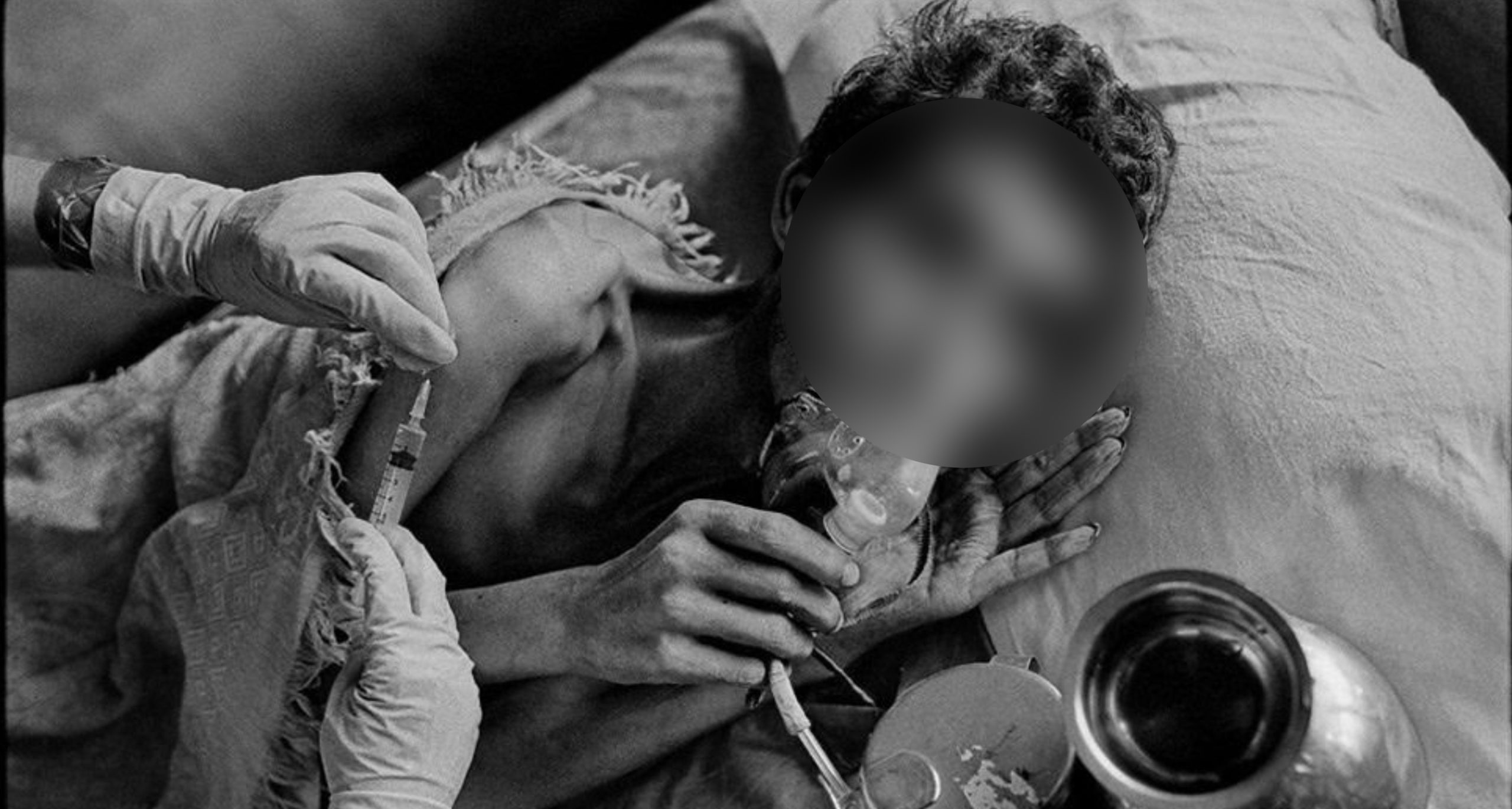
A patient with advanced pulmonary TB in a hospital in Mumbai, receives a daily injection and oxygen. Photo: James Nachtwey/flickr
On March 10, the Bombay High Court directed the Centre to pass an order by April 28 this year to issue a Compulsory License to two patented anti-tuberculosis drugs — Bedaquiline and Delamanid. This is to ensure continuous access to the two tuberculosis (TB) drugs for people living with multi drug resistant (MDR) and extremely drug resistant (XDR) tuberculosis in the country. At present, these patients face a shortage of these drugs in the country.
Compulsory License is issued when the government allows production/usage of a patent-protected invention. This can only be done effectively by issuing a government use compulsory license in accordance with the Patents Act, 1970 (Section 92 or Section 100).
It was in January this year when a public interest litigation (PIL) under Article 226 was filed before the Bombay High Court at Mumbai, Maharashtra, to ensure continuous access to the two patented and expensive TB drugs for people living with MDR and XDR tuberculosis in the country.
India has the world’s highest burden of tuberculosis, with an estimated 2.69 million new cases every year, notes a 2019 report of the World Health Organization (WHO). Every fourth MDR-TB patient is from India. The petitioners, through their counsel Anand Grover, pointed out that Mumbai has a high burden of multi and extremely drug resistant TB in the country.

The two patented drugs include Bedaquiline, manufactured by Janssen Pharmaceuticals, Inc., and Delamanid by Otsuka Pharmaceutical. The National Tuberculosis Elimination Programme heavily relies on donations of doses of these drugs by the companies to meet the public health demand.
But, these donations are miniscule compared to what is required. In 2019, the Indian government received 600 courses of Bedaquiline from Janssen followed by 22,000 courses from USAID [United States Agency for International Development]. Similarly, the programme received 400 courses of Delamanid as donation from Otsuka.
Meanwhile, the Central government has not been able to procure the two drugs as tenders have failed and drugs are being sold at high prices by Janssen and Otsuka. “Due to the price factor, the government has been dependent on donations, which are very limited. For a country with the highest TB burden in the world, you cannot depend on donations to run a national programme,” Brinelle D’Souza, a TB survivor and co-convener of Jan Swasthya Abhiyan, a non-profit working for public health based in Mumbai told Gaon Connection. She is one of the petitioners along with Mira Yadav, another TB survivor.
The two patented drugs are recommended by the WHO to treat TB patients more effectively. Moreover, as per the petition, the Subject Expert Committee of the Drugs Controller General of India (DCGI) has also approved their use under programmatic conditions, and the National Tuberculosis Elimination Programme has included them in the guidelines for the programmatic management of drug resistant tuberculosis in India. However, due to high cost and procurement problems, TB patients in the country face shortage of these drugs.
Bedaquiline is estimated to cost about Rs 26,600 for a six-month course. A multi drug resistant (MDR) and extremely drug resistant patient may require additional courses of the drug in case an extended regimen of 18 months is prescribed — this would cost Rs 79,800 for one patient. Delamanid, on the other hand, is estimated to cost about Rs 91,414 a patient for a six-month course.
As per the petition, Bedaquiline and Delamanid, together with repurposed drugs linezolid and clofazimine, provide opportunities to cure people with multi drug-resistant TB, prevent deaths, and the development of further resistance and reduce new infections.
“If medicines currently available are safe to use, the government must invoke compulsory licence provision and companies should manufacture drugs locally,” said D’Souza. This would also contribute towards the Central government’s target of eradicating tuberculosis by 2025, claim the petitioners.

